The FOx BIOSYSTEMS instrument is effective in studying affinity and binding kinetics of proteins on large particles, such as cells, viruses and microvesicles. The fluidics-free setup provides an advantage over existing biosensor platforms by eliminating the risk of clogging associated with cells and filamentous bacteria. In microfluidic instruments, this clogging necessitates time-consuming rinsing procedures and may impact accuracy.
Case study:
Bacteriophage kinetic affinity analysis
Increasing demands on biosensor sensitivity and specificity mean that multivalent bioreceptors are increasingly being used. In the field of bacteriophage affinity peptides, it is becoming more popular to use the entire phage as a receptor rather than the individual peptides. However, measuring the binding kinetics of complete phage particles is a challenge as these can clog microfluidics, albeit essential for the efficient design of these applications.
The FOx BIOSYSTEMS platform was used to study the affinity and binding kinetics of phage displaying peptide libraries in a microfluidic-free, dip-in experiment. A clear correlation was demonstrated between the SPR binding parameters and the expression of the target protein.
Case study:
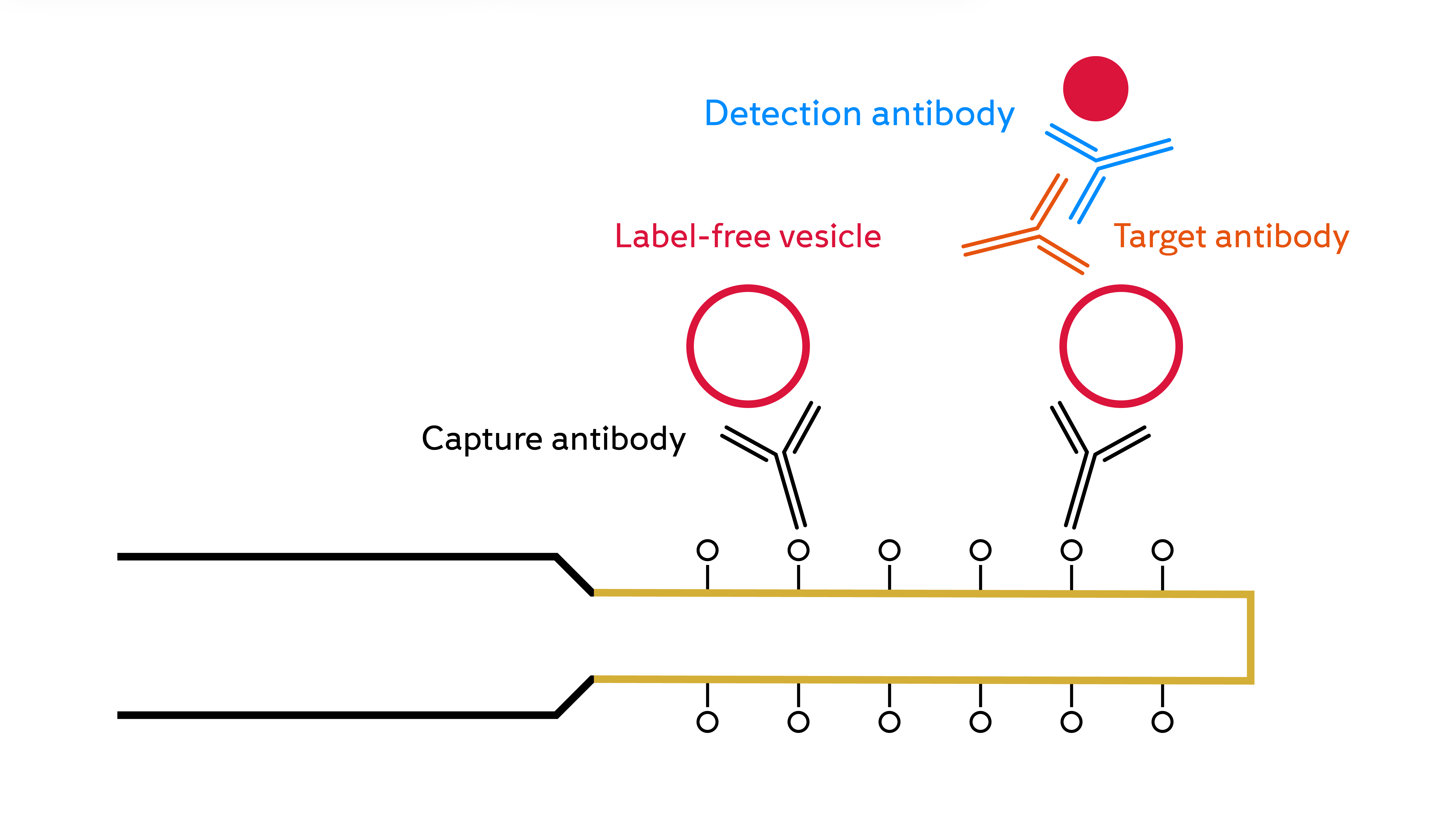
Detecting extracellular vesicles
Extracellular vesicles are becoming increasingly important in the diagnosis, prognosis and therapy of many diseases, including cardiovascular disease and cancer. Their analysis is complicated by their range of size, composition and origin, compounded by the complexity of biofluids. Currently available methods have a range of downsides, including inaccuracy, complex/high-cost instruments or inability to process complex matrices.
The FOx BIOSYSTEMS instrument provides a fast and accurate method of detecting extracellular vesicles at physiologically relevant sensitivities. Its sensitivity is 103 – 104 times higher than that required for detection in healthy or cancer patients’ plasma. It was also shown to selectively capture breast cancer EVs spiked in blood plasma on the FO-SPR surface.